NK-92 cells are a natural killer (NK) cell line derived from a patient with human malignant non-Hodgkin lymphoma. They were originally isolated from peripheral blood mononuclear cells of a 50-year-old Caucasian male patient. This cell line relies on interleukin-2 (IL-2) to maintain its proliferation and function, and will die within 72 hours in the absence of IL-2. NK-92 cells exhibit cytotoxicity against various malignant cells, particularly demonstrating killing ability against K562 and Daudi cells in chromium release assays.
NK-92 cell surface markers include positive expression of CD2, CD7, CD11a, CD28, CD45, and CD54, while CD1, CD3, CD4, CD5, CD8, CD10, CD14, CD16, CD19, CD20, CD23, CD34, and HLA-DR are negative. These cells grow in suspension with a doubling time of approximately 40-50 hours and are easy to culture and expand.
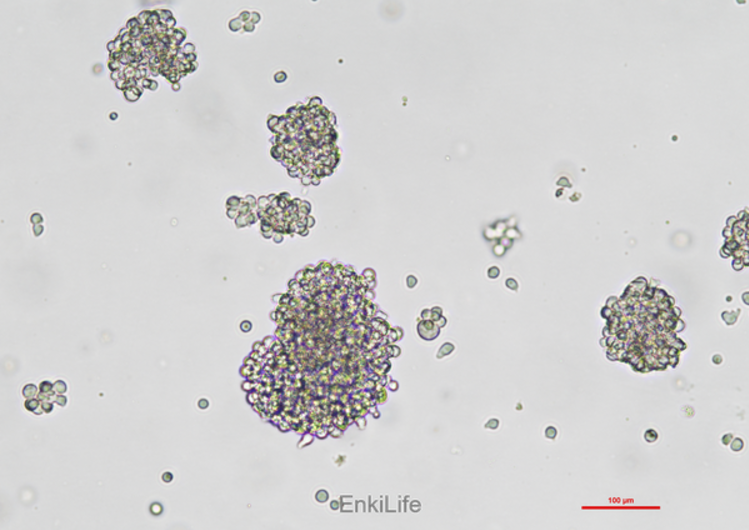
NK-92 cells are widely used in cancer, immunology, and toxicology research, and are the first NK cell line to receive FDA approval for clinical trials. However, due to their derivation from patients with malignant non-Hodgkin lymphoma, NK-92 cells carry potential risks of secondary tumors and susceptibility to Epstein-Barr virus. Therefore, radiation treatment is usually required before clinical application to ensure their safety [1,2]. In addition, NK-92 cells have also been genetically engineered, such as CAR-NK-92 cells, to enhance their anti-tumor activity [5,7].
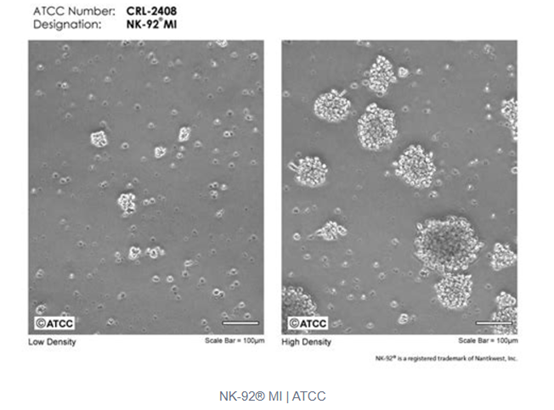
In summary, NK-92 cells, due to their unique origin and characteristics, possess significant potential for application in cancer treatment, but they also encounter certain challenges and limitations.
What are the clinical trial results of NK-92 cells in the treatment of human malignant non Hodgkin lymphoma?
The clinical trial results of NK-92 cells in the treatment of human malignant non Hodgkin lymphoma have shown certain potential, but the effectiveness is limited. NK-92 cells were isolated from peripheral blood mononuclear cells of a patient with invasive non Hodgkin lymphoma, exhibiting strong cytotoxicity and preventing malignant proliferation through radiation exposure [9].
Multiple Phase I clinical trials have studied the efficacy of NK-92 cell infusion after irradiation in patients with various hematological malignancies. Although these treatments have relatively low toxicity, their effectiveness is limited and may be related to insufficient persistence [11]. In addition, NK-92 cells have limitations in terms of expansion and persistence in vivo, as they originate from NK cell tumors and require radiation treatment to prevent malignant proliferation [11].
In a study, NK-92 cells were used to treat advanced cancer patients, showing mild adverse reactions and significant effects [3]. However, another study indicated that NK-92 cell therapy did not significantly prolong survival [8].
In addition, to overcome the limitations of NK-92 cells, researchers have attempted to enhance their function through engineering modifications. For example, high affinity NK cells (haNKs) have been developed through endogenous expression of IL-2 and high affinity CD16 receptors. These cells have been explored in various solid tumors, but are currently suspended in the QUILT 3.061 study of lymphoma [11].
What are the detailed information regarding the radiation treatment process of NK-92 cells and its impact on cell function?
NK-92 cells, a natural killer (NK) cell line derived from a lymphoma patient, are widely used in cancer immunotherapy due to their high cytotoxic activity against tumor cells and their "off-the-shelf" availability. However, because NK-92 cells themselves have a malignant origin, they require irradiation prior to clinical application to inhibit their proliferation and prevent potential tumorigenesis.
Irradiation Process
NK-92 cells are typically irradiated using gamma rays. In experiments, after culturing in specific medium, NK-92 cells are exposed to varying doses of gamma irradiation, ranging from 2.5 Gy to 20 Gy [10]. For instance, in one study, NK-92 cells were placed in a container with 15 ml of medium and irradiated using a cesium-137 source [10]. The radiation dose is selected based on simulations of clinical treatment conditions, with 10 Gy being a commonly used dose [14].
Impact of Irradiation on Cell Function
Cell Viability and Cytotoxic Function
Irradiation significantly impacts the viability and cytotoxic function of NK-92 cells. Studies show that as the radiation dose increases, the viability of NK-92 cells gradually decreases, while their cytotoxic function is also markedly reduced. For example, after 10 Gy irradiation, NK-92 cell viability significantly declines, and their serial killing capacity is reduced by more than two-thirds [10]. Specifically, unirradiated NK-92 cells exhibited a killing rate of 5.6 (KF 5.6) against targets, whereas NK-92 effector cells irradiated with 10 Gy showed a killing rate of only 1.5 (KF 1.5) [10].
Effect of Fas Receptor Activation
Fas (CD95) receptor activation plays an important role in irradiated NK-92 cells. Although NK-92 cells are insensitive to Fas ligand activation, anti-Fas treatment did affect non-necrotically irradiated NK-92 cells, manifesting as cell shrinkage [10]. Furthermore, irradiation increased the sensitivity of NK-92 cells to Fas ligand activation, leading to a significant decrease in their serial killing capacity [10].
Susceptibility to Immune System Attack
Irradiated NK-92 cells become more susceptible to attack by the patient's immune system. Studies indicate that irradiated NK-92 cells are more easily targeted by both unstimulated and IL-2-activated lymphokine-activated killer (LAK) cells [10]. In contrast, unirradiated NK-92 cells are more resistant to such attacks [10].
DNA Damage and Gene Expression Changes
Irradiation causes substantial DNA damage and leads to the accumulation of irradiated cells in the G2/M phase of the cell cycle. Transcriptome analysis revealed that gamma irradiation induced approximately 12 times more differentially expressed genes two hours post-irradiation compared to low-energy electron irradiation (LEEI) [14]. Surface molecule analysis showed a decrease in CD56 surface expression after irradiation, but no changes in the levels of activating receptors NKp46, NKG2D, or NKp30 [14].
Conclusion
Overall, irradiated NK-92 cells exhibit significantly reduced viability and cytotoxic function and are more vulnerable to immune system attack. These changes limit their effectiveness in in vivo therapy.
What are the comparative research results of CAR-NK-92 cells and traditional NK-92 cells in anti-tumor activity?
CAR-NK-92 cells exhibit significant advantages in anti-tumor activity compared to traditional NK-92 cells. Specifically:
1. Antitumor effect: Studies have shown that CAR-NK-92 cells have better anti-tumor activity against lymphoma cells in vivo, especially in CD19-CAR NK cells carrying IL-15/IL-15Ra, whose inhibitory effect on lymphoma cells in vivo is significantly better than that of traditional NK-92 cells [17]. In addition, research on breast cancer cells also shows that CAR modified NK cells show significant cytotoxicity to CD19 positive breast cancer cells, and can effectively activate and secrete a variety of cytokines, further enhancing their anti-tumor ability [18].
2. Persistence and stability: In some cases, CAR-NK-92 cells exhibit better persistence and stability. For example, in a prostate cancer model, irradiated dual CAR NK-92 cells exhibited comparable or even superior killing ability against tumor cells compared to the non irradiated control group [16]. This indicates that CAR-NK-92 cells have a stronger ability to maintain their function in vivo.
3. Wide application potential: CAR-NK-92 cells not only perform well in hematological malignancies, but also show great potential in solid tumor treatment. For example, the NK cell line YTS constructed with EGFRvIII specific CAR exhibited significant anti-tumor activity in the glioblastoma (GB) model, and by designing and producing CAR structures containing EGFRvIII specific antibodies MR1-1 and DAP12, the tumor detection and homing ability were improved, enhancing the tumor suppression effect [21].
4. Clinical application prospects: CAR-NK-92 cells have significant advantages over CAR-T cells, such as high cytotoxicity, rapid production of large numbers of cells, and avoidance of some targeted side effects, providing an alternative solution to the limitations of CAR-T cell therapy [7]. In addition, CAR-NK-92 cells have also shown less severe cytokine release syndrome or neurotoxicity in clinical trials, low-risk graft-versus-host disease, and safety of allogeneic NK cell infusion [20].
What are the roles of NK-92 cells in immune escape mechanisms and potential therapeutic strategies?
The role of NK-92 cells in immune escape mechanisms and their potential therapeutic strategies can be explored from multiple perspectives.
The role of NK-92 cells in immune escape
Multiple components in the tumor microenvironment (TME) regulate the expression of NKG2D receptors and NKG2DL ligands through different mechanisms, thereby affecting the function of NK cells. The deviation of NKG2D receptor/NKG2DL ratio from normal values can promote tumor escape from NK cell-mediated immune monitoring [26]. In addition, activation of the TGF β signaling pathway also inhibits the function of NK cells, leading to their loss of activity in the tumor microenvironment [28].
Cancer cells can evade attacks from NK cells through antigen loss or antigen unavailability. For example, downregulation of PD-L1 and ERBB2 expression may result in NK cells being unable to effectively recognize and kill tumor cells [27]. The downregulation of ICAM-1 is also a way for tumor cells to evade immune surveillance, and CAR-NK cells can overcome this obstacle by targeting these antigens [25].
There are various immunosuppressive factors in the tumor microenvironment, such as TGF β, IL-10, etc. These factors can inhibit the activity and metabolism of NK cells, making them unable to effectively combat tumors [28].
Potential treatment strategies
By genetically engineering NK-92 cells to express chimeric antigen receptors (CARs), their specific killing ability against tumor cells can be significantly improved. For example, bispecific CAR-NK cells can simultaneously target multiple antigens, thereby reducing the probability of antigen escape [16]. In addition, dual targeting CAR-NK cells targeting PD-L1 and ERBB2 can overcome immune escape caused by single target loss [27].
The Thea-Cot-NK92 system regulates its killing ability through coupling agents and can lyse cancer cells in vitro and in vivo models without the need for genetic modification of NK cells. This method is applicable to multiple antigens, saving the cost and time required for CAR reengineering [24].
Research has shown that by modifying the TGF β R2 signaling pathway, NK-92 cells can become insensitive to TGF β and enhance their anti-tumor properties [28]. In addition, by enhancing the metabolism and function of NK cells, such as using TGF β signaling inhibitors, the therapeutic effect can be improved [28].
NK cell therapy can be combined with other immunotherapy strategies such as CAR-T, CAR-NK, and anti-PD-1/PD-L1 targeting, as well as chemotherapy, radiotherapy, and tumor virus therapy strategies to achieve more comprehensive tumor elimination [26].
conclusion
NK-92 cells play an important role in the immune escape mechanism, mainly through immune suppressive factors, antigen loss, and antigen unavailability in the tumor microenvironment. The treatment strategies targeting these mechanisms include CAR-NK cell therapy, coupling agent mediated NK cell therapy, regulation of metabolism and signaling pathways, and combination therapy.
What are the safety and side effect management strategies for NK-92 cell therapy?
The safety and side effect management strategies of NK-92 cell therapy mainly include the following aspects:
1. Radiation treatment: NK-92 cells need to undergo radiation treatment before clinical application to reduce their potential risk of tumor formation and EB virus (EBV) infection. However, this radiation treatment can limit the proliferation and persistence of NK-92 cells in vivo, which may affect their long-term anti-tumor effects [30].
2. Safety in clinical trials: Multiple clinical trials have shown that NK-92 cells treated with radiation have good safety and tolerability in the treatment of advanced cancer patients. For example, in a trial targeting patients with kidney cancer and lung cancer, no serious adverse reactions were observed, with only one patient experiencing grade 3 fever and one patient experiencing grade 4 hypoglycemia events [34]. In addition, a phase I clinical trial targeting patients with acute myeloid leukemia did not report any grade 3-4 adverse events [33].
3. Preventive drug therapy: Prior to NK-92 cell infusion, preventive drugs are typically used to manage potential side effects. For example, in some studies, patients started taking specific drugs 2 hours before NK-92 cell infusion and took Allopurinol orally every day for the next 5 days to prevent cytokine release syndrome [12]. In addition, corticosteroids (such as Prednisone) and antihistamines (such as Clematin) are also used for preventive treatment [12].
4. Lymphatic castration chemotherapy: In order to improve the persistence of NK-92 cells, some studies suggest performing lymphatic castration chemotherapy before NK-92 cell infusion. This can overcome the immune system's rejection of NK cells by prioritizing the removal of lymphocytes from the recipient's body [33].
5. Cytokine support: Cytokines such as IL-2 and IL-15 are commonly used to support the survival and expansion of NK cells. However, the use of IL-2 may cause side effects such as discomfort, nausea, and vomiting, while IL-15 may increase immune fatigue and cytokine related toxicity [33].
6. Dose management: In clinical trials, the dose of NK-92 cells is usually adjusted according to the specific situation of the patient. For example, in a phase I/II study, patients received different doses of NK-92 cell therapy and decided whether to receive a second infusion based on the results of the first infusion [12].
In summary, the safety and side effect management strategies of NK-92 cell therapy involve multiple aspects such as radiation treatment, prophylactic drug therapy, lymphatic castration chemotherapy, cytokine support, and strict dose management.
References
1. The NK-92cell line—30 years later: its impact on natural killer cell research and treatment of cancer. [PMID: 36610812]
2. Natural Killer Cells: A Promising Kit in the Adoptive Cell Therapy Toolbox. Jiani Xiao et al.
[PMID: 36428748]
3. Gene-modified NK-92MI cells expressing a chimeric CD16-BB-ζ or CD64-BB-ζ receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Ying Chen et al. [PMID: 28415754]
4. Interaction kinetics with transcriptomic and secretory responses of CD19-CAR natural killer-cell therapy in CD20 resistant non-hodgkin lymphoma. Dashnamoorthy Ravi et al. [PMID: 31772298]
5. Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity. [PMID: 28572802]
6. Influence of Galectin-9 Treatment on the Phenotype and Function of NK-92MI Cells in the Presence of Different Serum Supplements. Matyas Meggyes et al. [PMID: 34439744]
7. Natural Killer Cells and Current Applications of Chimeric Antigen Receptor-Modified NK-92 Cell. [PMID: 30646574]
8. ErbB2 (HER2)-CAR-NK-92 cells for enhanced immunotherapy of metastatic fusion-driven alveolar rhabdomyosarcoma. Heim et al. [PMID: 37662907]
9. NK Cell-Based Immunotherapy for Hematological Malignancies. Simona Sivori et al. [PMID: 31623224]
10. Optimizing NK‑92 serial killers: gamma irradiation, CD95/Fas‑ligation, and NK or LAK attack limit cytotoxic efficacy. Navarrete Galvan et al. [PMID: 35366943]
11.Leveraging Natural Killer Cell Innate Immunity against Hematologic Malignancies: From Stem Cell Transplant to Adoptive Transfer and Beyond. Chenyu Lin et al. [PMID: 36613644]
12. Verfahrenstechnik fur die Kultivierung hamatopoetischer Zellen. Sebastian Schmidt et al.
13. Successful expansion and cryopreservation of human natural killer cell line NK-92 for clinical manufacturing. [PMID: 38394177]
14. Low Energy Electron Irradiation Is a Potent Alternative to Gamma Irradiation for the Inactivation of (CAR-)NK-92 Cells in ATMP Manufacturing. John Maher et al. [PMID: 34149724]
15. Reciprocal Complementation of the Tumoricidal Effects of Radiation and Natural Killer Cells. Kailin Yang et al. [PMID: 23634213]
16. Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells. [PMID: 35287669]
17. Engineering NK-CAR.19 cells with the IL-15/IL-15Rα complex improved proliferation and anti-tumor effect in vivo. [PMID: 37818365]
18. Continuously expanding CAR NK-92 cells display selective cytotoxicity against B-cell leukemia and lymphoma. [PMID: 27887866]
19. Current Perspectives on the Use of off the Shelf CAR-T/NK Cells for the Treatment of Cancer. [PMID: 33923528]
20. Preclinical and clinical studies of CAR-NK-cell therapies for malignancies. [PMID: 36353643]
21. Renaissance of armored immune effector cells, CAR-NK cells, brings the higher hope for successful cancer therapy. [PMID: 33752707]
22. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. [PMID: 30805309]
23. Anti-PSMA CAR-Engineered NK-92 Cells: An Off-the-Shelf Cell Therapy for Prostate Cancer. [PMID: 32498368]
24. A modifiable universal cotinine-chimeric antigen system of NK cells with multiple targets. [PMID: 36713381]
25. CAR-mediated targeting of NK cells overcomes tumor immune escape caused by ICAM-1 downregulation. [PMID: 38417916]
26. Natural killer group 2D receptor and its ligands in cancer immune escape. Shixin Duan et al. [PMID: 30813924]
27. 216 Dual targeting of CAR-NK cells to PD-L1 and ErbB2 facilitates specific elimination of cancer cells of solid tumor origin and overcomes immune escape by antigen loss. Jiri Eitler et al.
28. NK Cell Metabolism and TGFβ - Implications for Immunotherapy. [PMID: 31921174]
29. Cordycepin Sensitizes Cholangiocarcinoma Cells to Be Killed by Natural Killer-92 (NK-92) Cells. [PMID: 34641520]
30. Natural killer cells: the next wave in cancer immunotherapy. [PMID: 35967421]
21. Natural Killer Cells for Therapy of Leukemia. [PMID: 27226791]
32. Optimizing NK Cell-Based Immunotherapy in Myeloid Leukemia: Abrogating an Immunosuppressive Microenvironment. [PMID: 34220833]
33. Manipulating NK cellular therapy from cancer to invasive fungal infection: promises and challenges. [PMID: 36969979]
34. Emerging NK cell therapies for cancer and the promise of next generation engineering of iPSC-derived NK cells. [PMID: 35580928]
